Abstract submissions
The Organising Committee of the Oslo HEMS Conference 2025 invites you to submit original research abstracts for presentation at the upcoming conference, taking place in Oslo from 1st to 3rd December 2025. We encourage submissions from a broad range of fields relevant to prehospital care, including prehospital medicine, trauma, resuscitation, rescue techniques and translational research.
Publication
Submitted abstracts will be considered for publication in a conference supplement of the Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine (SJTREM) on an open access basi
Presentation
Accepted abstracts will be taken for presentation at the conference. The majority will be asked to present a poster. Prepare a poster in portrait orientation in A1 size (594 x 841 mm).
A small number of abstracts will be selected for oral presentation at “Holmgang”. Holmgang is an informal evening event where the author is given five minutes to present their work and five minutes for questions from the audience.
The authors will be notified which presentation they are selected for when receiving the Notification of Acceptance on September 26th. More information regarding Holmgang will be provided.
Submission details
Submit your abstract to: abstracs@ohemsc.no
Use the text “O-HEMS-C 2025 Abstract Submission” in the subject field.
By submitting this abstract you confirm that
- The abstract may be published in SJTREM
- The author´s email can be published along with the abstract
- The abstract is formatted according to the guidelines
- The study has all necessary ethical approvals
Important dates
- Abstract Submission Deadline: September 14th, 2025*
- Notification of Acceptance: September 26th, 2025
- Conference Dates: December 1th – 3rd, 2025
*Note that you are welcome to submit your work prior to this date.
Guidelines
File format
Please submit the abstract as a Microsoft Word-file (.DOC, .DOCX). Name the file with “O-HEMS-C 2025, Abstract, [your name]”, e.g.:
O-HEMS-C, Abstract, John Smith.DOCX
Language
UK or US English only.
Font
Times New Roman, font size 12.
Title
The title should be in bold, sentence case with no full stop at the end and no underlining,
Results from experiments in this field
Authors
First name, middle initials if required, and surname with no full stop at the end. Underline the name of the corresponding author. A comma should separate author names. Where authors are from a number of different institutions, the appropriate institution number from the affiliation list should be given as a superscript number immediately after each author’s name, e.g.:
John Smith1, Susan Jones1, Bill Fisher2
An asterisk (*) should be used to link the corresponding author with their email address.
If the authors are presenting an abstract on behalf of a study group, this information should not be included in the author list but should appear in the Acknowledgements section.
Affiliations
Affiliations should include department, institute, town, and country. Only provide one affiliation per author. Where there are multiple affiliations, each should be listed as a separate paragraph. Each institute should appear in the order used against the author names (see above paragraph) and show the appropriate superscript number, e.g.:
1Department, University, Town, State, USA
2University, Town, State, UK
3Company, Town, State, Canada
Main text
The abstract must be structured and not exceed 350 words, excluding title, authors and affiliations. Please minimise the use of abbreviations and do not cite references. Reports of randomized controlled trials should follow the CONSORT extension for abstracts (https://www.equator-network.org/reporting-guidelines/consort-abstracts/). The abstract must include the following separate sections:
- Background: the context and purpose of the study
- Methods: how the study was performed and statistical tests used
- Results: the main findings
- Conclusion: brief summary and potential implications
- Trial registration: If your article reports the results of a health care intervention on human participants, it must be registered in an appropriate registry and the registration number and date of registration should be stated in this section. If it was not registered prospectively (before enrolment of the first participant), you should include the words ‘retrospectively registered’.
Further, the abstract should adhere to the following:
- Single line spacing
- Type the text unjustified, without hyphenating words at line breaks
- Section headings (Background, Methods, Results, Conclusion, Trial registration) should be written in bold and no full stop or colon, only hard return
- Use hard returns only to end headings and paragraphs, not to rearrange lines
- Greek and other special characters may be included. If you are unable to reproduce a particular special character, please type out the name of the symbol in full
- SI units should be used throughout (litre and molar are permitted)
Tables and Figures
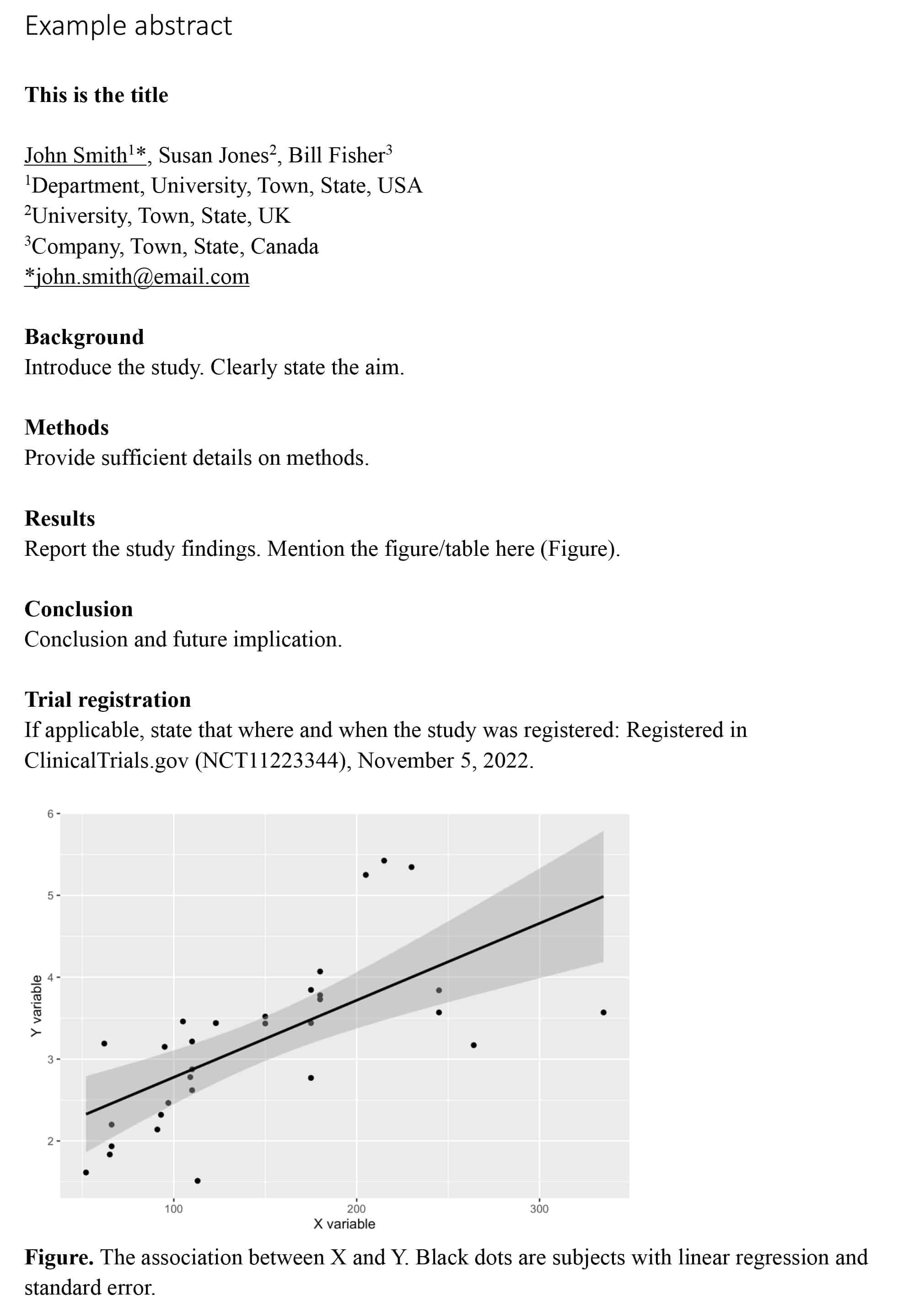
One figure or table is allowed. The figure or table should be placed after Conclusions(or Trial registration) and must be mentioned in the main text. Tables should be formatted using the “table function” in a word processing program, not created with tabs or submitted as graphical items. Tables should not have highlighting or shading. Tables should be submitted in editable format. Table titles should be placed below the table e.g.:
“Table. Short title, this is the text.”
Figures must be supplied electronically in the body of the text at 300 dpi minimum. The figure must be inserted as a single, composite file and not inserted into a table. Please align figures with text. Figure titles must form part of the text file and not be part of the graphical figure and placed below the figure, e.g.:
“Figure. Short title, must be separate, editable text and not embedded in image.”
Acknowledgements
Brief acknowledgements may be included and should be placed after Conclusions (or Trial registration).
Consent and ethics
Any abstract that includes identifiable information about individual patients must include a Consent to Publish statement as a separate file.
Research involving human subjects must have been approved by an appropriate ethics committee. Abstracts without necessary ethics and consent statements will not be accepted.
References
References are not allowed.
Example abstract